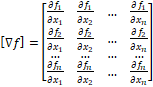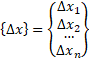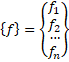COMBUSTION
AND FUELS WEEK 2
(ACR)
Chemical equilibrium
, Stoichiometric algorithm
Actual products formed in a reaction is not a clear
cut concept as we defined in the previous chapter. The actual products formed
in a chemical reactions can only be determined
experimentally and as a function of the environmental values such as
temperature, pressure and time. When these parameters changed actual products
can change as well. One way of estimating what products will be formed is to
use equilibrium concept. All physical systems are tend
to minimize their energy levels when there are no any external force or energy
to the system. For example a bubble takes spherical
shape under the influence of surface tension which has the minimal area for the
given volume. Chemical reactions when temperature and pressure is constant and
long enough time is given, tends to minimize it energy level, specifically
giibs free energy level (or maximize its entropy level). This state is called
chemical equilibrium. For example consider a closed system consisting initially
of a gaseous mixture of carbondioxide , oxygen and
carbonic acid. A reaction might take place is
![]()
![]()
![]()
At equilibrium the system will consist basically of
three components, CO2, H2CO3 and H2O,
for not all the components gases. This is what is called soda reaction. When
soda bottle opened equailibrium condition (pressured in the system) is changed
and rection slides to a new equilibrium states, so that some omount of CO2
and H2O
is formed and amount of carbonic acid is reduced . Changes in the amounts of these components
during the opening of soda bottle:
![]()
![]() (6.2.1) where dn denotes the differential change in
the representative component.
(6.2.1) where dn denotes the differential change in
the representative component.
![]() (6.2.2)
(6.2.2)
Equilibrium
is a condition of balance. When equilibrium is established amount of CO2
and H2O and H2CO3 tends to be balanced with
each other. But when equilibrium is broken and a new conditions arise, like
opening of a soda bottle, a new equilibrium will be established. And during
these process total gibbs free energy of the system will be minimised.
![]() (6.2.3) This amount
can be determined from chemical potential changes (see the first chapter for
details)
(6.2.3) This amount
can be determined from chemical potential changes (see the first chapter for
details)
![]() (6.2.4)
(6.2.4)
![]() (6.2.5)
(6.2.5)
This is called the
equilibrium reaction.For a more general equilibrium
equation such as:
![]()
![]()
![]() (6.2.6) where
(6.2.6) where ![]() s are stoichiometric coefficients. The following equilibrium
case is existed:
s are stoichiometric coefficients. The following equilibrium
case is existed:
![]() (6.2.7) where
(6.2.7) where ![]() is the proportionality factor. From which the following
expressions are obtained.
is the proportionality factor. From which the following
expressions are obtained.
![]()
![]()
![]()
![]() (6.2.8)
(6.2.8)
For this case the Gibbs free
energy equation takes form:
![]() (6.2.9)
(6.2.9)
For ideal gas mixtures
chemical potential can be expressed as specific gibbs free energy as
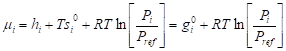 (6.2.10) where Pi is the partial pressure
of the gas. For and ideal gas, partial pressure can be expressed as a function
of components in the total components
(6.2.10) where Pi is the partial pressure
of the gas. For and ideal gas, partial pressure can be expressed as a function
of components in the total components
![]()
![]() . (6.2.11) Substituting these into the equation gives:
. (6.2.11) Substituting these into the equation gives:
![]() (6.2.12) Then the basic Gibbs free energy equation
becomes
(6.2.12) Then the basic Gibbs free energy equation
becomes
![]() (6.2.13)
(6.2.13)
Arranging equation:
![]() (6.2.14)
(6.2.14)
![]() (6.2.15)
(6.2.15)
This equation can be written
as:
![]() (6.2.16) or
(6.2.16) or
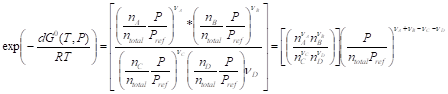 (6.2.17)
(6.2.17)
The left hand side of the
equation is called equilibrium constant, which is only function of temperature
for ideal gases.
![]() (6.2.18)
(6.2.18)
Equilibrium constant
evaluation is already defined in chemical Reaction class. As an example let us
evaluate equilibrium constant of the equilibrium reaction
![]()
![]()
![]()
At T=2000 K
chemicalReaction.java
|
import
java.io.*; import
java.util.*; import
javax.swing.*; import
java.awt.*; import
java.awt.event.*; public
class chemicalReaction {String
name[]; double
N[][]; Gas
g[]; int natom,ngas; int nrt,npt; String
reacName; double
A[][]; double
b0[]; double
b1[]; Atom
atomList[]; String
atomnames[]; String
atomsymbols[]; chemicalReaction(String
crname,String si[],double Ni[][]) {
reacName=crname; ngas=si.length; name=new
String[ngas]; N=new
double[ngas][2]; g=new
Gas[ngas]; for(int
i=0;i<ngas;i++) { name[i]=si[i]; g[i]=new
Gas(name[i]); } for(int
i=0;i<ngas;i++) {N[i][0]=Ni[i][0];N[i][1]=Ni[i][1];} arrange_atoms(); conservation(); } chemicalReaction(String
name) throws IOException { File
reacFile=new File("Reaction.txt"); int ngas; String tempGasName=""; try {
BufferedReader cfin=new
BufferedReader(new FileReader(reacFile)); int ierror=1; natom=0; try { while(cfin!=null) { reacName=Text.readString(cfin); if(reacName.equals(name))
{ierror=0;break;} } } catch(EOFException e_eof) { System.out.println("error
required gas mixture "+name+" is not found"); cfin.close();return; } ngas=Text.readInt(cfin); g=new
Gas[ngas]; N=new double[ngas][2]; nrt=0; npt=0; Double n1,n2; for(int i=0;i<ngas;i++) { tempGasName=Text.readString(cfin); N[i][0]=Text.readDouble(cfin); N[i][1]=Text.readDouble(cfin); nrt+=N[i][0]; npt+=N[i][1]; g[i]=new
Gas(tempGasName); } } catch(FileNotFoundException fnfe)
{System.out.println("File Not Found");} name=tempGasName; arrange_atoms(); //end of constructor conservation(); } public void arrange_atoms() { // atomic structure of the reactants int i,j; for(i=0;i<ngas;i++) {for(j=0;j<g[i].natom;j++) {add_atom(i,j);} } } public int add_atom(int i,int j) { //determine and order the atomic
structure int k; for(k=0;k<natom;k++) { if(g[i].atomList[j].symbol.equals(atomList[k].symbol)) { atomList[k]=new Atom(atomList[k].symbol,atomList[k].N+g[i].atomList[j].N*N[i][0]); return 1; } } Atom atomL[]; atomL=new Atom[natom+1]; for(k=0;k<natom;k++) atomL[k]=new Atom(atomList[k]); atomL[natom]=new Atom(g[i].atomList[j].symbol,g[i].atomList[j].N*N[i][0]); atomList=atomL; natom+=1; return 2; } public String toString() { // writes chemical reaction in
chemistry norm String s=""; for(int i=0;i<ngas;i++) { if(N[i][0]==1) { s+=g[i].toString(); } else
if(N[i][0]!=0) { s+=N[i][0]+"
"+g[i].toString(); } if(i!=(ngas-1) && N[i][0]!=0 )
s+=" + "; } if(s.endsWith(" + ")) s=s.substring(0,s.length()-3); s+="
--> "; for(int i=0;i<ngas;i++) { if(N[i][1]==1) { s+=g[i].toString(); } else
if(N[i][1]!=0) { s+=N[i][1]+"
"+g[i].toString(); } if(i!=(ngas-1) && N[i][1]!=0 )
s+=" + "; } if(s.endsWith(" + ")) s=s.substring(0,s.length()-3); return s; }
public String toStringE() { // writes chemical reaction in
chemistry equilibrium norm String s=""; for(int i=0;i<ngas;i++) { if(N[i][0]==1) { s+=g[i].toString(); } else
if(N[i][0]!=0) { s+=N[i][0]+" "+g[i].toString();
} if(i!=(ngas-1) && N[i][0]!=0 )
s+=" + "; } if(s.endsWith(" + ")) s=s.substring(0,s.length()-3); s+="
"+'\u21C4'+" "; for(int i=0;i<ngas;i++) { if(N[i][1]==1) { s+=g[i].toString(); } else
if(N[i][1]!=0) { s+=N[i][1]+"
"+g[i].toString(); } if(i!=(ngas-1) && N[i][1]!=0 )
s+=" + "; } if(s.endsWith(" + ")) s=s.substring(0,s.length()-3); return s; } public String toString(String ch) { //return the c String s=""; int i,j; if(ch.equals("name")) s=s+reacName+"\n"; else if(ch.equals("formula")) { s=toString(); } else if(ch.equals("composition")) { for(i=0;i<natom;i++) s=s+atomList[i].toString()+"
"; } return s; }
public double H(double TR,double TP) { // reaction enthalpy double HH=0; double h1,h2; for(int i=0;i<ngas;i++) { h1=g[i].ht(TP)*N[i][1]; h2=g[i].ht(TR)*N[i][0]; //System.out.println("h1="+h1+"h2="+h2);
HH+=h1; HH-=h2; } return HH; } public double S(double TR,double
TP,double pt) { //reaction entropy double TP1,TR1,pt1; double s1,s2; TR1=TR;TP1=TP;pt1=pt; double SS=0; for(int i=0;i<ngas;i++) { if(N[i][1]!=0)
SS+=g[i].si(TP1,N[i][1]/npt*pt1)*N[i][0]; if(N[i][0]!=0)
SS-=g[i].Si(TR1,N[i][0]/nrt*pt1)*N[i][1]; } return SS; } public double S(double TR,double TP) { //reaction entropy double TP1,TR1,pt1,pt; pt=1.0;TR1=TR;TP1=TP;pt1=pt; return S(TR,TP,pt); } public double G(double TR,double
TP,double pt) { // reaction gibbs energy double TP1,TR1,pt1; TR1=TR;TP1=TP;pt1=pt; double GG=0; for(int i=0;i<ngas;i++) { if(N[i][1]!=0) GG+=g[i].gt(TP1,N[i][1]/npt*pt1)*N[i][1]; if(N[i][0]!=0)
GG-=g[i].gt(TR1,N[i][0]/nrt*pt1)*N[i][0]; } return GG; } public double G(double TR,double TP) { // reaction gibbs energy double TP1,TR1,pt1,pt; pt=1.0;TR1=TR;TP1=TP;pt1=pt; return G(TR,TP,pt); } public double G0(double TR,double TP) { // reaction gibbs energy double GG=0; double h1=0; for(int i=0;i<ngas;i++) { if(N[i][1]!=0) { h1=g[i].gt(TP)*N[i][1];GG+=h1;} if(N[i][0]!=0) { h1=g[i].gt(TR)*N[i][0];GG-=h1;} } return GG; } public double K(double TR,double TP) { // Equilibrium costant. Usually TR and
TP will be in // the same temperature return Math.exp(G0(TR,TP)/(-8.3145*TP)); } public double lnK(double TR,double TP) { // Equilibrium costant. Usually TR and
TP will be in // the same temperature return G0(TR,TP)/(-8.3145*TP); }
public boolean conservation() { //Gives atomic mass balance of the chemical
reaction atomnames=new String[natom]; atomsymbols=new
String[natom]; boolean bb=true; int nn=ngas; int nat=g[0].atomList[0].atom_name.length; double B[][]=new double[nat][nn]; int nk=0; for(int i=0;i<nn;i++) { for(nk=0;nk<g[i].atomList.length;nk++){ B[g[i].atomList[nk].number-1][i]=g[i].atomList[nk].N; } } int natcount=0; boolean zeroflag[]=new boolean[nat]; for(int j=0;j<nat;j++) {
zeroflag[j]=false; for(int i=0;i<nn;i++) {if(B[j][i]!=0){zeroflag[j]=true;}}; if(zeroflag[j])
{atomnames[natcount]=g[0].atomList[0].atom_name[j];
atomsymbols[natcount]=g[0].atomList[0].atom_symbol[j]; natcount++;} } A=new double[natcount][nn]; int k=0; for(int j=0;j<nat;j++) {if(zeroflag[j]){A[k]=B[j];k++;}} natom=A.length; ngas=A[0].length; b0=new double[natom]; for(int ii=0;ii<natom;ii++) { for(int ij=0;ij<ngas;ij++) { double carpim=A[ii][ij]*N[ij][0]; b0[ii]+=carpim; } } b1=new double[natom]; for(int ii=0;ii<natom;ii++) { for(int ij=0;ij<ngas;ij++) { double carpim=A[ii][ij]*N[ij][1]; b1[ii]+=carpim; } } for(int ii=0;ii<natom;ii++) { if(b0[ii]==b1[ii])
bb=bb&&true; else bb=bb&&false; } return bb; } public void equilibrium_print(double
TR,double TP) { JTextArea jta; JPanel jpan=new JPanel(); jpan.setLayout(new
BorderLayout()); String s="Equilibrium Reaction
name :
"+toString("name")+"\n"; s+="Equilibrium formula : "+toStringE()+"\n"; s+="Treactant
= "+TR+" degree K\n"; s+="Tproduct = "+TP+ " degree K\n"; s+="Equilibrium
constant K = "+K(TR,TP)+"\n"; s+="Equilibrium
constant lnK = "+lnK(TR,TP)+"\n"; s+="Equilibrium
constant log10K = "+Math.log10(K(TR,TP))+"\n"; s+="Reaction
composition : "+toString("composition")+"\n"; s+="Atom
balance check :
"+conservation()+"\n";
s+="Atomic
balance:"; jta=new JTextArea(s); natom=A.length; ngas=A[0].length; String bb="Equilibrium Chemical
Reaction"; String baslik[]=new String[ngas+3]; String s1[][]=new String[natom][ngas+3]; int i,j; for(i=0;i<ngas;i++) {baslik[i+1]=g[i].gasName;} baslik[i+1]="b0(reactants)"; baslik[i+2]="b1(products)"; for(j=0;j<natom;j++) {s1[j][0]=atomsymbols[j];} for(j=0;j<natom;j++)
{ for(i=0;i<ngas;i++)
{s1[j][i+1]=""+A[j][i];} } for(j=0;j<natom;j++)
{s1[j][i+1]=""+b0[j]; s1[j][i+2]=""+b1[j]; } JTable jt; int n=A.length; int m=A[0].length; genelModel gm=new genelModel(s1,baslik); jt=new JTable(gm);
//JOptionPane.showMessageDialog(null,jt);
jpan.add(jta,BorderLayout.NORTH);
jpan.add(new JScrollPane(jt),BorderLayout.SOUTH); JFrame cerceve=new JFrame(bb); cerceve.addWindowListener(new
BasicWindowMonitor()); cerceve.getContentPane().add(new
JScrollPane(jpan)); cerceve.pack(); cerceve.setVisible(true); } public void print(double TR,double TP) { JTextArea jta; JPanel jpan=new JPanel(); jpan.setLayout(new
BorderLayout()); String s="Reaction name :
"+toString("name")+"\n"; s+="Reaction
formula :
"+toString("formula")+"\n"; s+="Reaction
composition : "+toString("composition")+"\n"; s+="Atom
balance check :
"+conservation()+"\n"; s+="Treactant
= "+TR+" degree K\n"; s+="Tproduct = "+TP+ " degree K\n"; double Taf=Tadiabatic(TR); s+="Adiabatic
flame temperature = "+Taf+"\n"; double Q1=H(298.15,298.15); s+="Heating
value = "+Q1+" kJ/kmol\n"; double Q2=H(TR,TP); s+="Q =
"+Q2+" kJ/kmol \n"; s+="heating
efficiency = "+Q2/Q1+"\n"; s+="\n"; s+="Atomic
balance:"; jta=new JTextArea(s); natom=A.length; ngas=A[0].length; String bb="Chemical Reaction"; String baslik[]=new String[ngas+3]; String s1[][]=new String[natom][ngas+3]; int i,j; for(i=0;i<ngas;i++) {baslik[i+1]=g[i].gasName;} baslik[i+1]="b0(reactants)"; baslik[i+2]="b1(products)"; for(j=0;j<natom;j++) {s1[j][0]=atomsymbols[j];} for(j=0;j<natom;j++)
{ for(i=0;i<ngas;i++)
{s1[j][i+1]=""+A[j][i];} } for(j=0;j<natom;j++)
{s1[j][i+1]=""+b0[j]; s1[j][i+2]=""+b1[j]; } JTable jt; int n=A.length; int m=A[0].length; genelModel gm=new genelModel(s1,baslik); jt=new JTable(gm);
//JOptionPane.showMessageDialog(null,jt);
jpan.add(jta,BorderLayout.NORTH);
jpan.add(new
JScrollPane(jt),BorderLayout.SOUTH); JFrame cerceve=new JFrame(bb); cerceve.addWindowListener(new
BasicWindowMonitor()); cerceve.getContentPane().add(new
JScrollPane(jpan)); cerceve.pack(); cerceve.setVisible(true); } public String atombalance() {String s=" |"; for(int i=0;i<name.length;i++)
{
if(name[i].length()==2) s+=name[i]+" "; else
if(name[i].length()==2) s+=name[i]+"
"; else
if(name[i].length()==3) s+=name[i]+" "; else
if(name[i].length()>=4) s+=name[i]; } s+="|b0 b1 |\n"; for(int j=0;j<atomList.length;j++)
{s+=atomsymbols[j]+"|"; for(int i=0;i<name.length;i++)
{s+=A[j][i]+" ";} s+="
|"+b0[j]+" "+b1[j]+"|\n"; } return s; } public double Tadiabatic(double TR) {
double Taf=Taf(TR,298.0,2000.0); if(Taf>1999.0&&Taf<=2000.0
) Taf=Taf(TR,298.0,3000.0); if(Taf>2999.9&&Taf<=3000.0)
Taf=Taf(TR,298.0,4000.0);
if(Taf>3999.9&&Taf<=4000.0) Taf=Taf(TR,298.0,5000.0); return Taf; } public double Taf(double TR,double
xl,double xu) { double TR1,xl1,xu1; TR1=TR;xl1=xl;xu1=xu; // Adyabatic flame temperature using
Bisection method // bisection method to find roots of
zero energy change // Q=0 // defination of variables
: // xl : lower
guess // xu : upper
guess // xr : root
estimate // es : stopping
criterion // ea :approximate
error // maxit : maximum
iterations // iter : number of iteration double test; double xr=0; double es,ea; double fxl=0,fxr=0; int maxit=500,iter=0; es=0.000001; ea=1.1*es; while((ea>es)&&(iter<maxit)) { xr=(xl1+xu1)/2.0; iter++; if((xl1+xu1)!=0) { ea=Math.abs((xu1-xl1)/(xl1+xu1))*100;} fxl= H(TR,xl1); fxr= H(TR,xr); test= fxl*fxr; if(test==0.0) ea=0; else
if(test<0.0) xu1=xr; else { xl1=xr; } } //end of while return xr; } double Vi(double TR,double TP,int Ni) {return -G(TR,TP)/(96487*Ni);} double Vi0(double TR,double TP,int Ni) {return -G0(TR,TP)/(96487*Ni);} } |
reactionTest7.java
|
import
javax.swing.*; import
java.io.*; public
class reactiontest7 { public
static void main(String arg[]) throws IOException { String
s[]={"co","o2","co2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0}}; double
Tproduct=3000.0; // degree K double
Treactant=3000.0; // degree K chemicalReaction
cr=new chemicalReaction("CO equilibrium",s,N); cr.equilibrium_print(Treactant,Tproduct); } } |
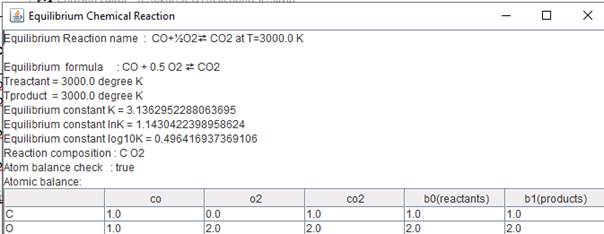
Now
we can use this knowledge to calculate an equilibrium composition. For a better
understanding of the process, we would like to solve an example problem by
hand, before establishing a computer solution. One kmol of CO an done kmol of O2 established an equilibrium at
3000 K. The equilibrium reaction for this is as follows:
![]()
![]()
![]()
The
reaction will be
CO+O2àn0CO+n1O2+n2CO2
Find
the equilibrium composition. System pressure is P=1.01325 bar. (Pref=1.01325
bar)
The
reaction will be
CO+O2àn0CO+n1O2+n2CO2
Find
the equilibrium composition.
Atomic
balance for C: 1=n0+n2
Atomic
balance for O: 3=n0+2n1+2n2
Equilibrium
constant at T=3000 K :
K=3.1362952
![]()
![]()
![]()
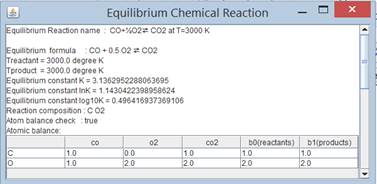
![]()
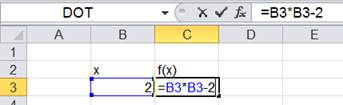
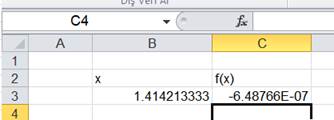
If this equation is solved by excel solver
|
n0=nCO |
0.335081662 |
|
n1=nO2 |
0.667540831 |
|
n2=NCO |
0.664918338 |
|
ntotal |
1.667540831 |
|
lnK |
1.143042231 |
|
|
-1.143042319 |
|
equation |
-8.789E-08 |
|
In
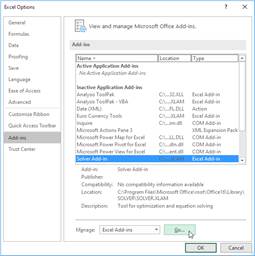
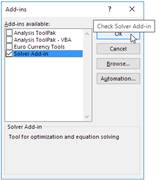
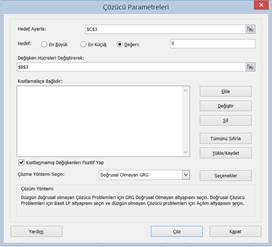
excel, solver is existed to find real roots: We can use solver add in. In
order to open solver: On the File tab, click Options. (in turkish
version dosya, seçenekler) Under Add-ins(in turkish version eklentiler),
select Solver Add-in (in turkish version Çözücü eklentisi), and click on the Go(Git..)
button. You can find the Solver on the Data tab(Veri), in the Analyze
group.
|
Now
the same solution can be carried out by computer program. In order to solve the
system a system of equation solver is used. For this purpose continuity method
is used.
Below
listed continuity method (it is also called homotophy method) to solve non-linear
system of equation. This method is relatively less dependent to initial
estimation of the system of equation solution, therefore a good selection for
solving the system of non-linear equation. The method details is as follows:When a problem of system of nonlinear equations of
the form F(x)=0 desired to be solved, assume that solution set to be found is
x*. Consider a parametric function G(l,x) in
the form of
G(l,x) = lF(x) + (1-l)[ F(x) - F(x(0)) ]
Where l=0
coresponds to initial guess of the solution , x(0)
,and where l=1 value corresponds the actual solution
set x(1)= x*
It is desired to be found G(l,x) = 0 therefore for l=0 equation becomes
G(l,x) =G(0,x) = F(x) - F(x(0)) and for l=1
0=G(1,x)
= F(x)
Therefore at x(1)=x* solution
set will be obtained. If a function G(l,x)
satisfies the above equation can be found,
it will also find us the solution. Function G is called a homotopy
between the function G(0,x) and G(1,x)=F(x). In order to find such a function,
it is assumed to have a function
G(l,x)=0 is
existed and partial derivative of this function with respect to l and x will also be zero
![]()
if x(l) is
isolated form this equation, it becomes:
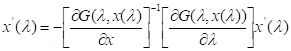
If G(l,x) = lF(x) + (1-l)[ F(x) - F(x(0)) ] equation is substituted into the differential
equation
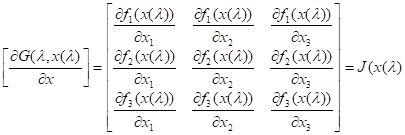
Forms a Jacobian matrix. and
![]()
Differential equation becomes
![]()
It is
possible to solve such a differential equation by using initial value problem
approaches, solution at x(1) will be given us the roots of the system of
equation. Solutions of initial value problems will be given latter chapters in
details, but A sixth order Runge-Kutta differential equation solution will be
defined here to solve our homotopy problem. If equation
![]() is given the 6th order
Runge-Kutta method to numerically solve this differential equation is defined
as:
is given the 6th order
Runge-Kutta method to numerically solve this differential equation is defined
as:
yi+1
= yi + (1/90)*( 7k1 + 32k3 +12k4+32k5+7k6)h
k1=f(xi,yi)
k2=f(xi+0.25h , yi+0.25k1h)
k3=f(xi+0.25h , yi+0.125k1h+0.125k2h)
k4=f(xi+0.5h , yi 0.5k2h+k3h)
k5=f(xi+0.75h , yi + (3/16)k1h+(9/16)k4h)
k6=f(xi+h , yi - (3/7)k1h+(2/7)k2h+(12/7)k3h
- (12/7)k4h+(8/7)k5h)
This
equation can be given as Buthcher tableu as:
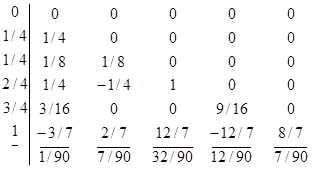
In these equations h is
finite difference step size. Solution starts by using the initial value l=0 , x0(0) and adds h into l in each
iteration step. The code given here uses 6th degree Runge-Kutta method to solve
homotopy(Continuation problem). It should be note that Homotophy method is less dependent to
initial value compare to methods such as Newton-Raphson therefore one
possibility is to approach solution with a relatively rough estimate with
homotophy following with a Newton-Raphson type of method, which is quite
efficient when the estimation approaches the correct roots. In the following code methods of
Homotophy with 6th order Runge-Kutta methods and combination of Homotophy with
4th order Runge-Kutta and Newton-Raphson method is given.
if_xj.java interface
|
@FunctionalInterface interface ifi_xj { // multifunction multi independent variable // vector of dependent variables are
returned // example f[0]=x[0]+sin(x[1]) //
f[1]=x[0]*x[1]-x[1] // func(x) returns the value of f[0] and
f[1] // as a two dimensional vector public double[] func(double x[]); default
double[] xp(double x[],double j,double m,double h) { //h
= dx //j=-4..4 multiplication factor //m
derivative taken value int
k=x.length; double
xx[]=new double[k]; for(int
i=0;i<k;i++) {
if(i==m) xx[i]=x[i]+j*h; else xx[i]=x[i]; } return
xx; } default
double[][] dfunc(double x[]) { double
h=1.0e-12; return
dfunc(x,1,h); } default
double[][] dfunc(double x[],int n) { double
h=1.0e-12; return
dfunc(x,n,h); } default
double[][] dfunc(double x[],int n,double h) { int
k=x.length; //number of x values (dimension) double
df[][]=new double[k][k]; for(int
i=0;i<k;i++) {
for(int j=0;j<k;j++) {df[i][j]=dfunc(x,n,i,j,h);} } return
df; } default
double dfunc(double x[],int n,int j,int m) { double
h=1.0e-12; return
dfunc(x,n,j,m,h); } default
double dfunc(double x[],int n,int j,int m,double h) {
// derivative of x[m] //m derivative taken value //jth equation //nth derivative of the equation (n=0)
function value //n=1 first derivative n=2 second
derivative.. int
k=x.length; //number of x values (dimension) double
hh=1/h; double
ff[][]=new double[9][3]; double
xp[][]=new double[9][3]; double
df; int
j1; double
x1=0; x1=x[m]; for(int jj=-4;jj<=4;jj++) {
j1=jj+4; xp[j1]=xp(x,jj,m,h); ff[j1]=func(xp[j1]); } if(n==0)
{df=ff[4][j];} else if(n==1) {
df=(3.0*ff[0][j]-32.0*ff[1][j]+168.0*ff[2][j]-672.0*ff[3][j]+672.0*ff[5][j]-168.0*ff[6][j]+32.0*ff[7][j]-3.0*ff[8][j])/840.0*hh;} else if(n==2) {df=(-14350.0*ff[4][j]-9.0*ff[0][j]+128*ff[1][j]-1008*ff[2][j]+8064*ff[3][j]+8064.0*ff[5][j]-1008.0*ff[6][j]+128.0*ff[7][j]-9.0*ff[8][j])/5040.0*hh*hh;} else if(n==3) {df=(-7.0*ff[0][j]+72.0*ff[1][j]-338.0*ff[2][j]+488.0*ff[3][j]-488.0*ff[5][j]+338.0*ff[6][j]-72.0*ff[7][j]+7.0*ff[8][j])/240.0*hh*hh*hh;} else if(n==4) {df=(2730.0*ff[4][j]+7.0*ff[0][j]-96.0*ff[1][j]+676.0*ff[2][j]-1952.0*ff[3][j]-1952.0*ff[5][j]+676.0*ff[6][j]-96.0*ff[7][j]+7.0*ff[8][j])/240.0*hh*hh*hh*hh;} else if(n==5) {df=(ff[0][j]-9.0*ff[1][j]+26.0*ff[2][j]-29.0*ff[3][j]+29.0*ff[5][j]-26.0*ff[6][j]+9.0*ff[7][j]-ff[8][j])/6.0*hh*hh*hh*hh*hh;} else if(n==6) {df=(-150.0*ff[4][j]-ff[0][j]+12.0*ff[1][j]-52.0*ff[2][j]+116.0*ff[3][j]+116.0*ff[5][j]-52.0*ff[6][j]+12.0*ff[7][j]-ff[8][j])/4.0*hh*hh*hh*hh*hh*hh;} else if(n==7) {df=(-ff[0][j]+6.0*ff[1][j]-14.0*ff[2][j]+14.0*ff[3][j]-14.0*ff[5][j]+14.0*ff[6][j]-6.0*ff[7][j]+ff[8][j])/2.0*hh*hh*hh*hh*hh*hh*hh;} else if(n==8) {df=(70.0*ff[4][j]+ff[0][j]-8.0*ff[1][j]+28.0*ff[2][j]-56.0*ff[3][j]-56.0*ff[5][j]+28.0*ff[6][j]-8.0*ff[7][j]+ff[8][j])*hh*hh*hh*hh*hh*hh*hh*hh;} else df=0; return
df; } } |
Function f_equailibriumi.java
|
import
java.io.*; import
java.util.*; import
javax.swing.*; import
java.awt.*; import
java.awt.event.*; public
class f_equilibriumi implements ifi_xj {
public double n0[]; public double n[]; int nn,n1,n2; String name[]; double TR,TP; public chemicalReaction cr; public double A[][]; public double b[]; public Gas g[]; double Pref=1.01325;//bar double P; chemicalReaction chr[]; public f_equilibriumi(double n0i[],String namei[],
chemicalReaction chri[],double T1i,double T2i,double Pi) { nn=n0i.length; int nn1=namei.length; if(nn!=nn1) System.out.println("array
size error nn="+nn+"nn1="+nn1); P=Pi; n2=chri.length; n1=nn-n2; n0=new double[nn]; n=new double[nn]; g=new Gas[nn]; name=new
String[nn]; TR=T1i; TP=T2i; chr=new chemicalReaction[n2]; for(int i=0;i<n2;i++) {chr[i]=chri[i];} for(int i=0;i<nn;i++) { n0[i]=n0i[i]; name[i]=namei[i]; g[i]=new
Gas(name[i]); } double N[][]=new double[nn][2]; for(int i=0;i<nn;i++) {
N[i][0]=n0[i]; N[i][1]=n[i]; } cr=new chemicalReaction("base
reaction",name,N); cr.conservation(); A=cr.A; b=cr.b0; } public double[] func(double n[]) { double b1[]=new double[nn]; double nt=0; for(int i=0;i<nn;i++) {nt+=n[i];} for(int ii=0;ii<n1;ii++) { for(int ij=0;ij<nn;ij++) { double carpim=A[ii][ij]*n[ij]; b1[ii]+=carpim; } b1[ii]=b1[ii]-b[ii]; } double lnK; double total; double mu[][]=new double[nn][2]; double nx[]; for(int ii=n1;ii<nn;ii++) { int
ij=ii-n1; int nn2=chr[ij].ngas; nx=new double[nn2]; for(int i=0;i<nn;i++) {
for(int j=0;j<nn2;j++) {
if(name[i].equals(chr[ij].name[j])) {
mu[i][0]=chr[ij].N[j][0];mu[i][1]=chr[ij].N[j][1]; nx[j]=n[i]; break; } } } total=0; for(int i=0;i<chr[ij].ngas;i++) { if(nx[i]!=0) {total-=mu[i][0]*Math.log(nx[i]/nt*P/Pref); total+=mu[i][1]*Math.log(nx[i]/nt*P/Pref); } } lnK=chr[ij].lnK(TR,TP); b1[ii]=lnK-total; } return b1; } public void equilibrium_print(double ni[]) { double x[]=new double[nn]; double x0[]=new double[nn]; double nnt=0; double nn0t=0; for(int i=0;i<nn;i++){nnt+=ni[i];nn0t+=n0[i];} for(int i=0;i<nn;i++){x[i]=ni[i]/nnt;x0[i]=n0[i]/nn0t;} JTextArea jta; JPanel jpan=new JPanel(); jpan.setLayout(new
BorderLayout()); String s=""; s+="Treactant
= "+TR+" degree K\n"; s+="Tproduct = "+TP+ " degree K\n"; s+="P = "+P+" bar\n"; s+="==========================================\n";
for(int i=0;i<n2;i++) { s+="Equilibrium
Reaction : "+chr[i].toStringE()+"\n"; s+="Equilibrium
constant K = "+chr[i].K(TR,TP)+"\n"; s+="Equilibrium
constant lnK = "+chr[i].lnK(TR,TP)+"\n"; s+="==========================================\n"; } //for(int j=0;j<nn;j++) //{s+="n_"+name[j]+" =
["+n0[j]+" ,
"+ni[j]+" ]\n";} jta=new JTextArea(s); String
heading[]={"name","n0 mole in","x0 mole ratio
in","n mole out","x mole ratio out"}; String s1[][]=new String[nn+1][5]; for(int i=0;i<nn;i++) {s1[i][0]=name[i]; s1[i][1]=""+n0[i]; s1[i][2]=""+x0[i]; s1[i][3]=""+ni[i]; s1[i][4]=""+x[i]; } s1[nn][0]="total"; s1[nn][1]=""+nn0t; s1[nn][2]=""+1; s1[nn][3]=""+nnt; s1[nn][4]=""+1; JTable jt; genelModel gm=new genelModel(s1,heading); jt=new JTable(gm); jpan.add(jta,BorderLayout.NORTH); jpan.add(new
JScrollPane(jt),BorderLayout.SOUTH); String bb="Equilibrium Chemical
Reaction"; JFrame cerceve=new JFrame(bb); cerceve.addWindowListener(new
BasicWindowMonitor()); cerceve.getContentPane().add(new
JScrollPane(jpan)); cerceve.pack(); cerceve.setVisible(true); } |
Continuityi.java
|
import
java.util.*; import
java.awt.*; import
java.applet.Applet; import
java.awt.event.*; import
javax.swing.*; public
class continuityi { //
(Continuity-homotopy) method to solve a system of nonlinear equations public
static double[] multiply(double left,double[] right) { //multiplying
a vector with a constant int
i; int
n=right.length; double
b[]; b=new double[n]; for(i=0;i<n;i++) {b[i]=left*right[i];} return
b; } public
static double[] multiply(double[][] left,double[]
right) { //multiplication
of one matrix with one vector int
ii,jj,i,j,k; int
m1=left[0].length; int
n1=left.length; int
m2=right.length; double[]
b; b=new double[m2]; if(n1 != m2) { System.out.println("inner
matrix dimensions must agree"); for(ii=0;ii<n1;ii++) { b[ii]=0; } return b; } for(i=0;i<m1;i++) { b[i]=0; for(k=0;k<n1;k++) b[i]+=left[i][k]*right[k]; } return
b; //end
of multiply of a matrix and a vector } public
static double[] add(double[] left,double[] right) { //addition
of two vectors int
n1=left.length; int
n2=right.length; int
nMax; int
i; if(n1>=n2)
nMax=n1; else nMax=n2; double
b[]; b=new double[nMax]; for(i=0;i<n1;i++) { b[i]=b[i]+left[i]; } for(i=0;i<n2;i++) { b[i]=b[i]+right[i]; } return
b; //end
of vector addition method } public
static double[][] inv(double[][] a) { //
INVERSION OF A MATRIX //
inversion by using gaussian elimination //
with full pivoting int
n=a.length; int
m=a[0].length; double
b[][]; b=new double[n][n]; int
indxc[]; int
indxr[]; double
ipiv[]; indxc=new
int[n]; indxr=new
int[n]; ipiv=new
double[n]; int
i,j,k,l,ll,ii,jj; int
icol=0; int
irow=0; double
big,dum,pivinv,temp; if(n!=m) {
System.out.println("Matrix must be square
"); for(ii=0;ii<n;ii++) for(jj=0;jj<n;jj++) b[ii][jj]=0.0; return b; } for(i=0;i<n;i++) for(j=0;j<n;j++) b[i][j]=a[i][j]; for(i=0;i<n;i++) { big=0.0; for(j=0;j<n;j++) { if(ipiv[j] != 1) for(k=0;k<n;k++) { if(ipiv[k] == 0) { if(Math.abs(b[j][k])
>= big) { big=Math.abs(b[j][k]); irow=j; icol=k; } } else
if(ipiv[k] > 1 ) { System.out.println("error
: inverse of the matrix : singular matrix-1"); for(ii=0;ii<n;ii++) for(jj=0;jj<n;jj++) b[ii][jj]=0.0; return b; } } } ++ ipiv[icol]; if(irow != icol) for(l=0;l<n;l++) { temp=b[irow][l]; b[irow][l]=b[icol][l]; b[icol][l]=temp; } indxr[i]=irow; indxc[i]=icol; if(b[icol][icol] == 0.0) { System.out.println("error
: inverse of the matrix : singular matrix-2"); for(ii=0;ii<n;ii++) for(jj=0;jj<n;jj++) b[ii][jj]=0.0; return b; } pivinv=1.0/b[icol][icol]; b[icol][icol]=1.0; for(l=0;l<n;l++) b[icol][l] *=pivinv; for(ll=0;ll<n;ll++) if(ll != icol) { dum=b[ll][icol]; b[ll][icol]=0.0; for(l=0;l<n;l++) b[ll][l]-= b[icol][l]*dum; } } for(l=n-1;l>=0;l--) { if(indxr[l] != indxc[l]) for(k=0;k<n;k++) { temp=b[k][indxc[l]]; b[k][indxc[l]]=b[k][indxr[l]]; b[k][indxr[l]]=temp; } } return
b; }
public
static double[] continuationRK6(ifi_xj f,double x[],int N) { //==================================================== //
Roots of nonlinear system of equations Homotophy RK6 //
yi+1 = yi + (1/90)*( 7k1 + 32k3 +12k4+32k5+7k6)h //
k1=f(xi,yi) //
k2=f(xi+0.25h , yi+0.25k1h) //
k3=f(xi+0.25h , yi+0.125k1h+0.125k2h) //
k4=f(xi+0.5h , yi - 0.5k2h+k3h) //
k5=f(xi+0.75h , yi + (3/16)k1h+(9/16)k4h) //
k6=f(xi+h , yi - (3/7)k1h+(2/7)k2h+(12/7)k3h -
(12/7)k4h+(8/7)k5h) //=================================================== //x
vector of independent variables //y
vector of dependent variables //dy
derivative vector of dependent variables int
i; int
nmax=1000; double
tolerance=1.0e-15; int
n=x.length; double
h=1.0/(double)N; double
b[]=new double[n]; double
x1[]=new double[n]; double
k[][]=new double[6][n]; double
A[][]=new double[n][n]; b=multiply(-h,f.func(x)); for(i=0;i<N;i++) { A=f.dfunc(x); //
k1=f(xi,yi) k[0]=multiply(inv(A),b); x1=add(x,multiply(0.25,k[0])); A=f.dfunc(x1); //
k2=f(xi+0.25h , yi+0.25k1h) k[1]=multiply(inv(A),b); x1=add(x,add(multiply(0.125,k[0]),multiply(0.125,k[1]))); A=f.dfunc(x1); //
k3=f(xi+0.25h , yi+0.125k1h+0.125k2h) k[2]=multiply(inv(A),b); x1=add(x,add(multiply(-0.5,k[1]),k[2])); A=f.dfunc(x1); //
k4=f(xi+0.5h , yi - 0.5k2h+k3h) k[3]=multiply(inv(A),b); x1=add(x,add(multiply((3.0/16.0),k[0]),multiply((9.0/16.0),k[3]))); A=f.dfunc(x1); //
k5=f(xi+0.75h , yi + (3/16)k1h+(9/16)k4h) k[4]=multiply(inv(A),b); x1=add(x,
add(multiply((-3.0/7.0),k[0]),add(multiply((2.0/7.0),k[1]), add(multiply((12.0/7.0),k[2]),
add(multiply((-12.0/7.0),k[3]),multiply((8.0/7.0),k[4])))))); A=f.dfunc(x1); //
k6=f(xi+h , yi - (3/7)k1h+(2/7)k2h+(12/7)k3h -
(12/7)k4h+(8/7)k5h) k[5]=multiply(inv(A),b); //
yi+1 = yi + (1/90)*( 7k1 + 32k3 +12k4+32k5+7k6)h for(int
j=0;j<n;j++)
{x[j]=x[j]+1.0/90.0*(7.0*k[0][j]+32.0*k[2][j]+12.0*k[3][j]+32.0*k[4][j]+7.0*k[5][j]);} } return
x; } public
static double[] newton_continuationRK6(ifi_xj f,double x[]) { //
Roots of nonlinear system of equations Homotophy RK4 plus Newton-Raphson //ti : weight function //x
vector of independent variables //y
vector of dependent variables //dy
derivative vector of dependent variables x=continuationRK6(f,x,20); double
ti=1.0; int
i; int
nmax=1000; double
tolerance=1.0e-15; int
n=x.length; double
b[]; b=new double[n]; for(i=0;i<n;i++) { b[i]=1.0; } i=0; while(
i++ < nmax && Matrix.abs(b) >
tolerance ) {
b=Matrix.multiply(Matrix.divide(f.func(x),f.dfunc(x)),-ti);
x=Matrix.add(x,b); } if(i
>= nmax) JOptionPane.showMessageDialog(null,"Warning
: Maximum number of iterations are exceeded \n"+ " Results may not be
valid","MAXIMUM ITERATIONS WARNING",JOptionPane.WARNING_MESSAGE); System.out.println(); return
x; } public
static double[] newton(ifi_xj f,double x[]) { //
Roots of nonlinear system of equations Homotophy RK4 plus Newton-Raphson //ti : weight function //x
vector of independent variables //y
vector of dependent variables //dy
derivative vector of dependent variables double
ti=1.0; int
i; int
nmax=1000; double
tolerance=1.0e-15; int
n=x.length; double
b[]; b=new double[n]; for(i=0;i<n;i++) { b[i]=1.0; } i=0; while(
i++ < nmax && Matrix.abs(b) >
tolerance ) {
b=Matrix.multiply(Matrix.divide(f.func(x),f.dfunc(x)),-ti);
x=Matrix.add(x,b); } if(i
>= nmax) JOptionPane.showMessageDialog(null,"Warning
: Maximum number of iterations are exceeded \n"+ " Results may not be
valid","MAXIMUM ITERATIONS WARNING",JOptionPane.WARNING_MESSAGE); System.out.println(); return
x; } public
static double[] enterdata() { String s=JOptionPane.showInputDialog("enter
initial root estimations with a space in between "); StringTokenizer token=new
StringTokenizer(s); int n=token.countTokens()-1; int m=n+1; double a[]=new double[m]; int j=0; while(token.hasMoreTokens()) { Double ax=new Double(token.nextToken()); a[j++]=ax.doubleValue(); } return a; } public static void main(String arg[]) {
String s[]={"co","o2","co2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0}}; chemicalReaction r[]=new
chemicalReaction[1]; r[0]=new
chemicalReaction("r1",s,N); double Tproduct=3000.0;//degree K double Treactant=3000.0;//degree K double n0[]={1,1,0}; double P=1.01325;//bar f_equilibriumi fe=new
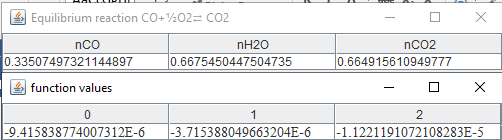
f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.3,0.3,0.3}; double [] r1= continuationRK6(fe,n,4); String
ss[]={"nCO","nH2O","nCO2","nH2","nO2"}; String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n"; double r2[]=fe.func(r1); Text.print(r1,ss,ss1); Text.print(r2,"function
values"); } } |
Of
course other non-linear system solving methods are also available
|
1 NEWTON-RAPHSON
METHOD One
dimensional Newton-Raphson Formula was:
If the
equation format changed a little, it can be written in the form of
Now
the same equation can be considered for a system of non-linear equation
So
multidimensional Newton-Raphson equation becomes
The
equation can be solved by using tecqniques such as gauss elimination. An
initial estimate for all the x values are required to start
iterative solution |
|
//
newtoni Newton Raphson method //
to solve non-lineer system of equation import
java.util.*; import
java.awt.*; import
java.applet.Applet; import
java.awt.event.*; import
javax.swing.*; //=============
Tanimi gereken fonksiyon ================ public
class newtoni { //
Roots of system of non-lineer equations //
By using Newton-Raphson Method public
static double[] newtond( ifi_xj f,double x[]) { //x
vector of independent variables //y
vector of dependent variable //dy
derivative of vector of dependent variable //
derivative vector calculated by numerical derivatives double
ti=1.0; int
i,ii,jj; int
nmax=500; double
tolerance=1.0e-15; int
n=x.length; double
b[]; b=new double[n]; double
dy[][]; dy=new
double[n][n]; i=0; for(i=0;i<n;i++) { b[i]=1.0; } while(
i++ < nmax && Matrix.abs(b) >
tolerance ) { b=Matrix.multiply(Matrix.divide(f.func(x),f.dfunc(x)),-ti); x=Matrix.add(x,b); } if(i
>= nmax) JOptionPane.showMessageDialog(null,"Maximum
number of iterations are exceeded \n"+ " Results might not be
valid","MAXIMUM NUMBER OF ITERATIONS ERROR",JOptionPane.WARNING_MESSAGE); return
x; } public
static double[] verigir() { String s=JOptionPane.showInputDialog("ENTER
FIRST ROOT ESTIMATES WİTH ONE EMPTY SPPACE IN BETWEEN: "); StringTokenizer token=new
StringTokenizer(s); int n=token.countTokens()-1; int m=n+1; double a[]=new double[m]; int j=0; while(token.hasMoreTokens()) { Double ax=new Double(token.nextToken()); a[j++]=ax.doubleValue(); } return a; } public static void main (String
args[]) {String
s[]={"co","o2","co2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0}}; chemicalReaction r[]=new
chemicalReaction[1]; r[0]=new
chemicalReaction("r1",s,N); double Tproduct=3000.0;//degree K double Treactant=3000.0;//degree K double n0[]={1,1,0}; double P=1.01325;//bar f_equilibriumi fe=new
f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.3,0.1,0.3}; double [] r1= newtond(fe,n0); System.out.println(Matrix.toString(r1)); /* String
ss[]={"nCO","nH2O","nCO2","nH2","nO2"}; String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n"; double r2[]=fe.func(r1); Text.print(r1,ss,ss1); Text.print(r2,"function
values"); */ } } |

The
same result is established in here as well. Now if conditions change, a new
equilibrium condition will result. If the pressure applied in this problem
becomes P=2 bar, the new equilibrium condition will be:
|
public
class equilibriumi {public
static void main(String arg[]) {
String s[]={"co","o2","co2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0}}; chemicalReaction r[]=new
chemicalReaction[1]; r[0]=new
chemicalReaction("r1",s,N); double Tproduct=3000.0;//degree K double Treactant=3000.0;//degree K double n0[]={1,1,0}; double P=2.0;//bar f_equilibriumi fe=new
f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.5,0.5,0.5}; double [] r1= continuityi.continuationRK6(fe,n,4); String
ss[]={"nCO","nH2O","nCO2","nH2","nO2"}; String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n"; double r2[]=fe.func(r1); Text.print(r1,ss,ss1); Text.print(r2,"function
values"); } } |
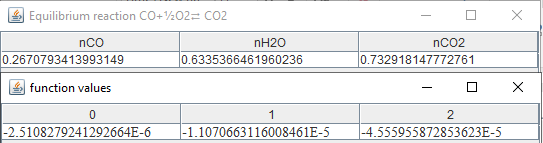
If
the temperature drops to 2500 K for a pressure P=1.01325 bar:
|
public class equilibriumi {public static void main(String arg[]) { String
s[]={"co","o2","co2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0}};
chemicalReaction r[]=new chemicalReaction[1]; r[0]=new chemicalReaction("r1",s,N); double Tproduct=2500.0;//degree K double Treactant=2500.0;//degree K double
n0[]={1,1,0}; double
P1.01325;//bar f_equilibriumi fe=new
f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.5,0.5,0.5}; double [] r1= continuityi.continuationRK6(fe,n,4); String ss[]={"nCO","nH2O","nCO2","nH2","nO2"}; String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n"; double r2[]=fe.func(r1); Text.print(r1,ss,ss1); Text.print(r2,"function
values"); } } |
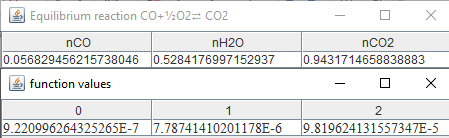
If
the temperature drops to 2000 K for a pressure P=1.01325 bar:
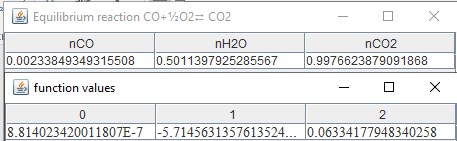
As
you can see
from the results, CO vanishes when equilibrium temperature gets lower.
For
T=3000 K and P=1.01325 bar, if N2 is added to the reaction. Equilibrium
reaction is still the same:
![]()
![]()
![]()
The
reaction will be
CO+O2+1.88N2àn0CO+n1O2+n2CO2+1.88N2
Find
the equilibrium composition.
Atomic
balance for C: 1=n0+n2
Atomic
balance for O: 3=n0+2n1+2n2
Equilibrium
constant at T=3000 K :
K=3.1362952
![]()
![]()
![]()
![]() l
l
If this equation is solved by excel solver
|
n0=nCO |
0.417734523 |
|
n1=nO2 |
0.708867261 |
|
n2=nCO |
0.582265477 |
|
n3=nN2 |
1.88 |
|
ntotal |
3.588867261 |
|
lnK |
1.143042231 |
|
|
-1.143042181 |
|
equation |
5.00389E-08 |
|
public class equilibriumi {public static void main(String arg[]) { String
s[]={"co","o2","co2","n2"}; double
N[][]={{1.0,0.0},{0.5,0.0},{0.0,1.0},{0,0}};
chemicalReaction r[]=new chemicalReaction[1]; r[0]=new chemicalReaction("r1",s,N); double
Tproduct=3000.0;//degree K double
Treactant=3000.0;//degree K double
n0[]={1,1,0,1.88}; double
P=1.01325;//bar f_equilibriumi fe=new
f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.5,0.5,0.5,1.88}; double [] r1= continuityi.continuationRK6(fe,n,4); fe.equilibrium_print(r1); } } |
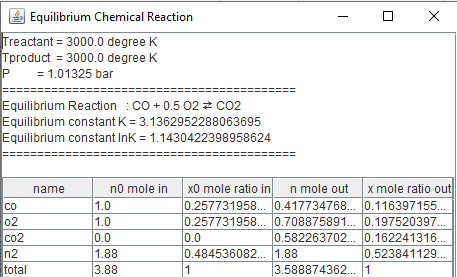
As
it is seen from the example, inert gas that does not goes into the reaction,
still changes the equilibrium composition.Now let us
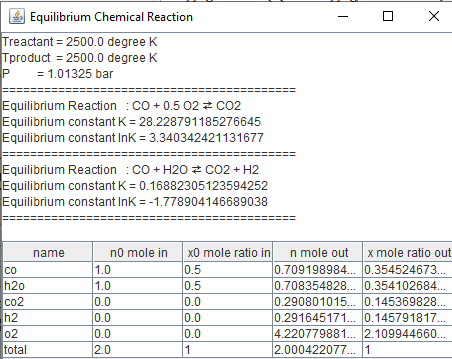
look at more than one simultaneous reactions going on together:One mole of CO
and one mole of H2O are heated to 2500 K and 1.01325 bar.
Determine the equilibrium composition if it is assumed that only CO, CO2,
H2, H2O and O2 are presented.
|
public
class equilibrium1i {public
static void main(String arg[]) {
chemicalReaction r[]=new chemicalReaction[2]; String
s[]={"co","h2o","co2","h2","o2"}; double
N1[][]={{1.0,0.0},{0,0},{0,1},{0.0,0.0},{0.5,0.0}}; r[0]=new
chemicalReaction("r0",s,N1); double
N2[][]={{1.0,0.0},{1.0,0.0},{0.0,1.0},{0.0,1.0},{0,0}}; r[1]=new
chemicalReaction("r1",s,N2); double Tproduct=2500.0;//degree K double Treactant=2500.0;//degree K double n0[]={1.0,1.0,0.0,0.0,0.0}; double P=1.01325;//bar
f_equilibriumi fe=new f_equilibriumi(n0,s,r,Treactant,Tproduct,P);
double n[]={0.5,0.5,0.5,0.5,0.5};
double [] r1= continuityi.newton_continuationRK6(fe,n);
String
ss[]={"nCO","nH2O","nCO2","nH2","nO2"};
String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n";
ss1+="CO+"+"at T="+Tproduct+" K ";
fe.equilibrium_print(r1); } } |
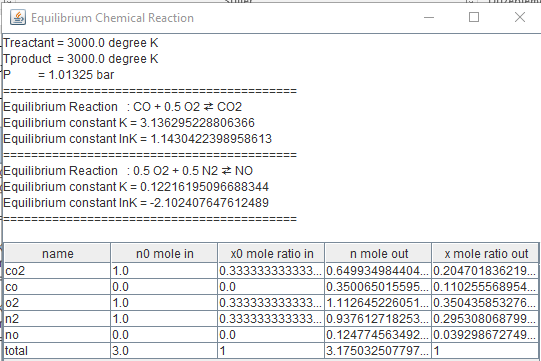
As a result of heating, a system consisting initially
of 1 kmol of CO2, kmol of O2, and kmol of N2
forms an equilibrium mixture of CO2, CO, O2, N2,
and NO at 3000 K, 1.01325 bar. Determine the composition of the equilibrium
mixture.
|
public class equilibrium2i {public static void main(String
arg[]) { chemicalReaction r[]=new
chemicalReaction[2]; String s[]={"co2","co","o2","n2","no"}; double
N1[][]={{.0,1.0},{1,0},{0.5,0},{0.0,0.0},{0.0,0.0}}; r[0]=new chemicalReaction("r0",s,N1); double
N2[][]={{0.0,0.0},{0.0,0.0},{0.5,0.0},{0.5,0.0},{0,1}}; r[1]=new chemicalReaction("r1",s,N2); double
Tproduct=3000.0;//degree K double
Treactant=3000.0;//degree K double
n0[]={1.0,0.0,1.0,1.0,0.0}; double
P=1.01325;//bar f_equilibriumi fe=new f_equilibriumi(n0,s,r,Treactant,Tproduct,P); double n[]={0.5,0.5,0.5,0.5,0.5}; double [] r1= continuityi.newton_continuationRK6(fe,n); String ss[]={"nCO","nH2O","nCO2","nH2","nO2"}; String ss1="Equilibrium reaction
CO+"+'\u00BD'+"O2"+'\u21C4'+" CO2 \n"; ss1+="CO+"+"at
T="+Tproduct+" K "; fe.equilibrium_print(r1); } } |
ÖDEV PROBLEMLERİ
HW1: Water at 3000 K and P=1.01325 bar pressure
dissociate to form some hydrogen and oxygen.
Calculate amount of O2 and H2
when systems came into equilibrium condition. Carry out this problem by hand
and by using computer program
HW2: As a result of heating, a system consisting
initially of 1 kmol of CO2, 1 kmol of O2, and 3.76 kmol
of N2 forms an equilibrium mixture of CO2, CO, O2,
N2, NO and NO2 at 3000 K, 1.01325 bar. Determine the
composition of the equilibrium mixture. Carry out this problem by hand and by
using computer program